Citrus vs the salt of the earth
by LJ Venter & Markus van Renssen (Horticulturists, Agri Technovation)
The challenge of salinization
Soil salinization presents a serious challenge in areas with a semi-dry climate. Saline soils lead to a variety of complications, especially in citrus, which could have a direct or indirect impact on the producer’s pocket.
Where soils are irrigated with high-salinity water (high EC), the crop’s production potential decreases with each irrigation, even if the effect is not immediately visible. High sodium levels in the soil react with soil colloids, causing a nutrient imbalance on the soil colloids which leads to soil dispersion. Soil dispersion prevents water from effectively filtering to the root zone and could also cause soil erosion. Soil crusting, poor drainage and compaction are all challenges associated with soils that have dispersed.
Water is one of the most important substrates in the photosynthesis process. It is also the resource that every plant needs to grow. In citrus, high sodium levels in the water cause several negative physiological effects on the plants.
Impact of soil salinization on the soil and citrus production
When sodium molecules dominate the soil colloids, it causes an imbalance in the availability of the other important cations in the soil – mainly calcium, magnesium and potassium. Said cations are displaced from the soil colloid and leached from the root zone by the flow of water. Sodium dominates the soil colloids as well as the ground-water suspension, which suppresses the uptake of the other cations.
Citrus has only moderate-to-low resistance to high-salt conditions. Sodium and chlorine cause damage to citrus trees through ion toxicity and osmotic pressure on roots.
Sodium and chlorine ion toxicity
The green pigment, chlorophyll, which captures light energy, is an important organelle used for photosynthesis, even in small green fruitlets. Sodium and chlorine ion toxicity refers to the build-up of these ions in a localized part of any plant organ. If the concentration of these ions becomes too high, it causes that portion of the plant organ to burn.
As a result, this necrotic part of the plant organ can no longer perform the necessary function (photosynthesis), and the tree’s potential to produce and accumulate carbohydrates is thereby reduced. This type of necrosis is usually observed on the leaf tips because sodium and chlorine move with water through the plant, thus translocating to the leaf tips. (Figure 1).

Figure 1: Example of a leaf tip that is necrotic – the affected leaf cannot photosynthesize optimally.
Roots undergo similar ion toxicity, causing root hairs to burn and wither. Ion toxicity can cause burn on up to 30% of each leaf, or even for the entire leaf to drop. Root hairs affected by sodium toxicity dry out and lose full function, drastically lowering the volume of water and nutrients absorbed by the plant.
The potential for carbohydrate production, uptake of essential elements (calcium, potassium, magnesium) and ultimately also crop potential, is therefore significantly reduced due to ion toxicity. Furthermore, high sodium that accumulates in fruit also causes rind defects such as splitting and creasing, resulting in a further negative impact on the producer’s crop potential.
Sodium and potassium are antagonistic in the plant, where high concentrations of sodium suppress potassium uptake (Kronzucker et al. 2013). Potassium controls the opening and closing of the guard cells of the stomata during transpiration. When the high sodium concentration suppresses potassium levels, less potassium is available for use by the guard cells. Sodium is incapable of controlling the regulation of the guard cells and, as a result, the tree transpires freely, resulting in water loss.
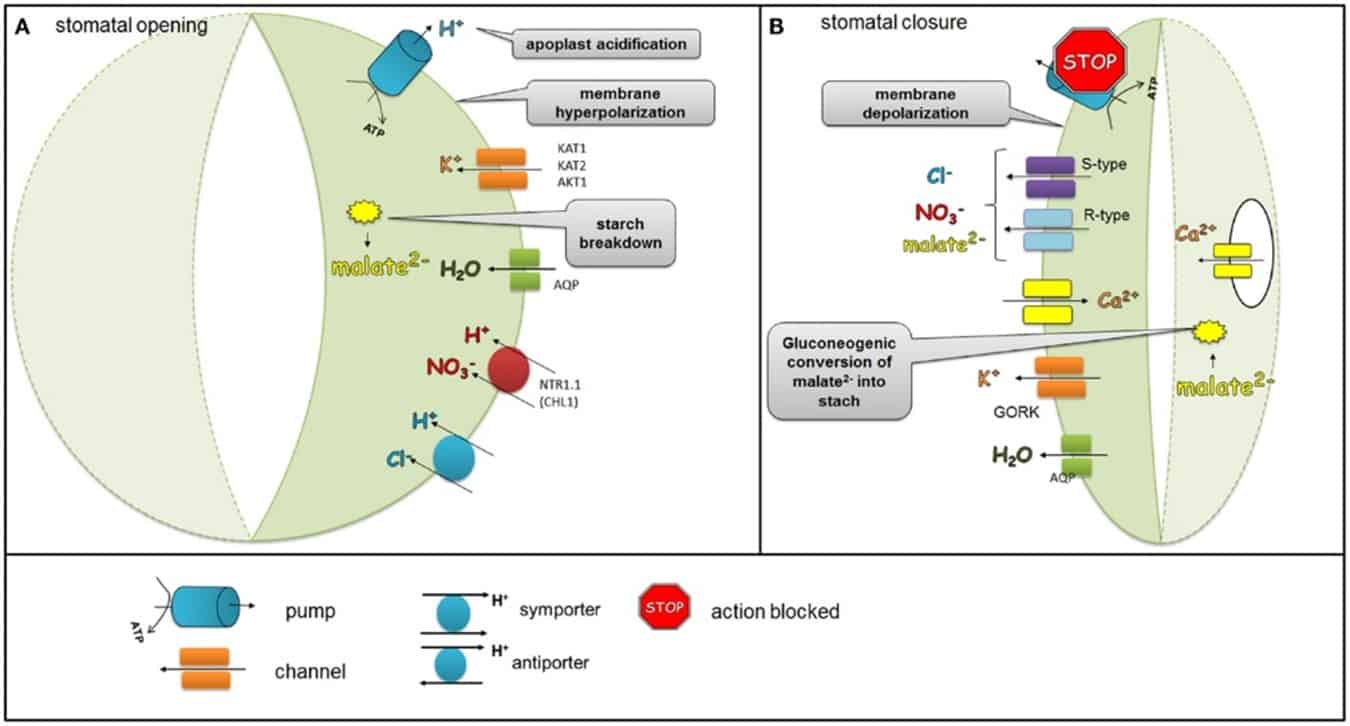
Most often, soil salinization is caused by high salinity irrigation water (high electrical conductivity or “EC”). The EC value depends on the water’s temperature and the dissolved salt concentration. A very high EC value in the water (EC > 1 mS.cm-1) is an indication that the water has a low osmotic potential (hypertonic) which ultimately affects the water potential (Ψ).
The osmotic potential of water is of great importance when it comes to the way in which water is absorbed into trees. The flow of water from the soil to the hair root is against the gradient of water potential, which means water moves from a low to a high water potential. In a healthy cycle where salinization is not a problem, the root cells contain more salt than the surrounding groundwater suspension. Thus, the water moves easily from the soil, through the root cell membrane into the root, where it is transported via the xylem into the plant.
This water translocation is driven by the osmotic pressure potential. However, in cases where the soil is salinized (or the water’s EC is constantly high), this process is hindered. To make it possible for water to still move into the root through the membrane, the tree needs to lower the roots’ water potential and does this by storing complex molecules such as carbohydrates and other nutrients in the roots. These carbohydrates are no longer available to be used for other metabolic processes in the tree, which results in a decrease of the tree’s production potential. If the soil’s EC becomes too high, water uptake will no longer be possible and the roots will experience ion toxicity.
Prevent high sodium levels
In the first instance, the sodium levels in the soil must be determined by accurate soil monitoring. By conducting regular ITEST™ SOIL precision mapping of the soil, sodium levels can be determined and monitored for timely correction. By using the MYFARMWEB™ Comparing Tool (function that makes it possible to compare maps) producers are able to compare the sodium levels in the soil over time and determine whether the concentration is rising or falling. If the levels increase, it is important to investigate further in order to identify the cause. Common causes of high sodium levels include the presence of sodium in irrigation water, underground water and also in the parent rocks.
Secondly, and because water quality fluctuates continuously, the sodium levels of irrigation water should be tested regularly through timely ITEST™ WATER analyses. If irrigation water is found not to be the cause of high sodium levels, then the following step would be to investigate the physical properties of the soil and the flow of underground water. Agri Technovation’s MYSOIL CLASSIFICATION™ service can be used to determine the physical properties of the soil, which could offer a possible solution to the salt-related problem.
Responsible management strategy for high-sodium soils
Once the cause of the high sodium levels has been identified, a strategy should be put in place to effectively address the problem. As mentioned above, salinization can be caused by a wide range of factors. Agri Technovation’s approach and focus is to tackle and solve each individual case with a unique, effective strategy. A few possible strategies are outlined below.
ITEST™ SOIL precision mapping and soil-chemistry corrections
Based on the ITEST™ SOIL precision chemical mapping, a precision correction map that focusses only on those areas where sodium levels are starting to become high, is generated. Gypsum is used to leach the sodium from the soil profile. When gypsum is applied to those areas where salts have accumulated, the following reaction will occur (Equation 1): Exchangeable salts (Na+) will bind with the sulphur in gypsum and calcium will swap seats with sodium on the soil colloid. Gypsum reacts with Na2CO3 as well as the absorbed sodium and is then leached out of the soil as sodium sulphate.
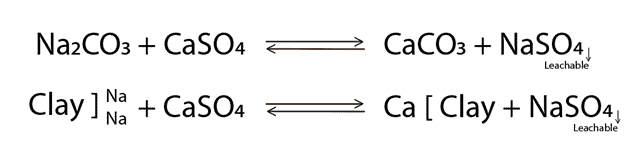
b. MYSOIL CLASSIFICATION™
In areas where a physical limiting layer in the soil causes poor drainage, the leaching of sodium from the soil profile will not be possible. Therefore, to ensure effective leaching, the limiting layer must first be broken before gypsum is applied. In areas where underground water is the sodium source, or where water accumulates underground, drainage should be installed to carry the water away. MYSOIL CLASSIFICATION™ soil maps are used in conjunction with ITEST™ SOIL precision maps to determine the most effective strategy for leaching the sodium.
c. Low-sodium fertilization strategy
The first step to successful sodium management is to solve the origin of the problem. If the problem is unavoidable, a sodium-friendly fertilizer programme should be
followed, in other words the programme will ensure that trees produce optimal
yields without adding unnecessary additional salts to the soil. NutriCAST™
plays an integral role in this programme. NutriCAST™ is an organic fertilizer
containing earthworm castings and is high in phosphorus, potassium, calcium and
magnesium. It also contains gypsum, which helps leach the sodium. By using NutriCAST™ instead of inorganic nitrogen, phosphorus, potassium and calcium fertilizers, the application of unnecessary additional salts to the soil is prevented.
Inorganic fertilizers will only be applied during critical phenological stages to target key processes. Rootfood Ca+S™, which acts as liquid gypsum, is also used in the fertilization programme to leach sodium from the root zone, thereby reducing the direct impact of salt on the tree’s roots and preventing ion toxicity as far as possible.
As explained by Y. Liang et al., (2007), silicon also plays a very important role in alleviating salt stress in plants, in that silicon:
Increases root activity, thereby increasing nutrient absorption;
Inhibits transpiration, thereby causing less osmotic stress; and
Increases ATPase and PPase enzyme activity in the tonoplast, promoting potassium uptake and thus suppressing sodium uptake.
By incorporating products such as Si – UNLOCK LPH™ (containing fulvic acid and orthosilicate) into the fertilization programme, sufficient silicon to address potential sodium stress is added. Fulvic acid increases the organic carbon concentration in the soil, which in turn stimulates hair root production and thus counteracts the sodium ion toxicity. The increase in organic carbon in the soil improves the natural buffering capacity and counteracts sodium uptake. This increases the release of cations from the clay particles in the soil, thereby effectively reducing the sodium in the suspension’s concentration.
As the build-up of salts in the root zone suppresses the uptake of important nutritional elements, it is essential that a complete foliar feeding programme is followed. During certain critical times the plant’s need for nutrients is more than what can be supplied by the roots. Salinization lowers the potential for nutrient uptake even further. It is therefore important to follow an effective foliar feeding programme that focuses on applying specific elements at the times when the tree needs them.
Timely intervention is the secret to success
Soil salinization therefore plays a significant role in water uptake, carbohydrate production, growth and ultimately the crop potential of the trees. Over the long term, the approach that involves following the steps and incurring the costs required to make timely corrections is more economical than the loss in yield and quality that could be suffered as a result of salinization. The success of effective sodium management lies in evaluating the specific situation timeously and then tackling the problem with a uniquely appropriate strategy.
References
1. Daszkowska-Golec,
A. and Szarejko, I. (2013) “Open or close the Gate – stomata action under
the control of phytohormones in drought stress conditions,” Frontiers in Plant Science, 4.
Available at: https://doi.org/10.3389/fpls.2013.00138.
2. Liang, Y. et
al. (2007) “Mechanisms of silicon-mediated alleviation of abiotic
stresses in higher plants: A Review,” Environmental Pollution, 147(2), pp. 422–428. Available at: https://doi.org/10.1016/j.envpol.2006.06.008.





