ZINC PHLOEM™: Improving Nutrient Delivery and Plant Physiological Status
By Rochelle Thuynsma, Head of Products: Technical
Role of Zinc in Plant Physiology
Plant growth and development depend on a sufficient supply of zinc (Zn). Photosynthesis, the main metabolic pathway in plants, can be significantly reduced by Zn deficiency, leading to a decrease in photosynthetic capacity, chlorophyll content, and stability.(1)
This reduction results in lower carbohydrate production, negatively affecting plant growth, particularly during reproductive stages. Zinc is also crucial for plant hormone production, especially auxins, which stimulate root and stem elongation.(2) Additionally, Zn acts as an important cofactor in the respiratory chain, where carbohydrates are consumed to produce biomass.(3) The concentration of water-soluble Zn in leaves is closely correlated with chlorophyll levels. The positive influence of foliar Zn fertilizer on photosynthesis and chlorophyll synthesis can help increase the absorption and accumulation of mineral nutrients in functional mature leaves.(4)
Chemistry of Zn
Foliar application of Zn is an effective way to address Zn deficiency.(2) However, the source of Zn being applied is critically important. Zinc has a strong capability for fixation in the cell wall following foliar application of ZnSO4 and Zn (NO3)2, which limits Zn penetration and reduces the effectiveness of the application.(5) This is due to the ionic nature of ZnSO4 and Zn (NO3)2, which dissociate into cations (Zn2+) and anions (SO4²⁻/NO3⁻) in water.(6)
Zinc mainly exists in a bound form in the cytoplasm and other cellular compartments to avoid uncontrolled Zn2+ binding to non-target sites.(6) Various studies have shown that the abundance of negatively charged sites in the cell wall limits the translocation of positively charged Zn2+. For example, the major component of pectin in cell walls is polygalacturonic acid, which has a high binding capacity for Zn2+.(5) Generally, there is low potential for the remobilization of foliar-absorbed nutrients until the binding sites within the leaf are saturated.(2)
Complexing Agents in ZINC PHLOEM™
Complexing agents refer to the binding of a metal/ion to an organic molecule via weaker binding forces to one or several ionic groups on the compound.(7) In this instance, the metal/ion is more available to dissociate from the binding.(8) The use of various natural complexes for foliar application of Zn enables rapid absorption and transfer of Zn through the stomata and epidermal pores of plant leaves.(9)
Organic acids are complexing agents that increase uptake efficiency and enhance plant assimilation.(7,9) These smaller molecules easily enter through the cuticle and stomata, have no net charge, and optimally diffuse through the leaf structure.(10) The organic nature of these compounds also ensures easy phloem loading and unloading, reducing the adenosine triphosphate (ATP) cost for nutrient transport and assimilation, increasing nutrient use efficiency.(10,11) The resulting molecules, after nutrient cleavage, can feed directly into plant metabolism, replenishing various metabolic intermediates and serving as a source of carbon.(11)
Nutritional Synergists in ZINC PHLOEM™
Various plant nutrients can affect the uptake and assimilation of other nutrients, ultimately influencing plant nutrient use efficiency.(12)
Nutrients can be classified as synergistic, antagonistic, or having no effect in relation to their interaction with other nutrients.(12) In formulating fertilizers, antagonistic nutrient interactions should be avoided, while synergistic nutrient interactions should be maximized for optimal nutrient use efficiency.(12)
ZINC PHLOEM™ is formulated with nutritional synergists to optimize nutrient delivery.
Nutritional synergists in ZINC PHLOEM™ can promote stomatal opening, providing more entry points for Zn to enter the leaf tissue. They can also act as a surfactant, reducing the surface tension of the foliar spray and allowing the applied solution to spread more evenly and penetrate the leaf cuticle more effectively.
Efficacy of ZINC PHLOEM™
ZINC PHLOEM™ is an organically complexed, foliar-applied Zn formulation, formulated with nutritional synergists to enhance uptake and assimilation. Complexing organic acids in ZINC PHLOEM™ ensure efficient leaf uptake, as well as facilitating phloem loading and unloading. This increases the movement of nutrients from source to sink organs and facilitates the assimilation of nutrients within sink tissues.
The efficacy of ZINC PHLOEM™ applications can be observed when compared to other market-related Zn-containing products. ZINC PHLOEM™ was effective in increasing foliar Zn levels to the same Zn level at half the applied concentration of the competitor product (Figure 1). ZINC PHLOEM™ is also readily taken up in waxy, thick cuticle leaves, such as those of macadamia trees (Figures 2A, 2B), where the application of ZINC PHLOEM™ increased both the leaf tissue concentration and water-soluble leaf Zn levels after application on day 0 and day 38 (Figures 2A, 2B).
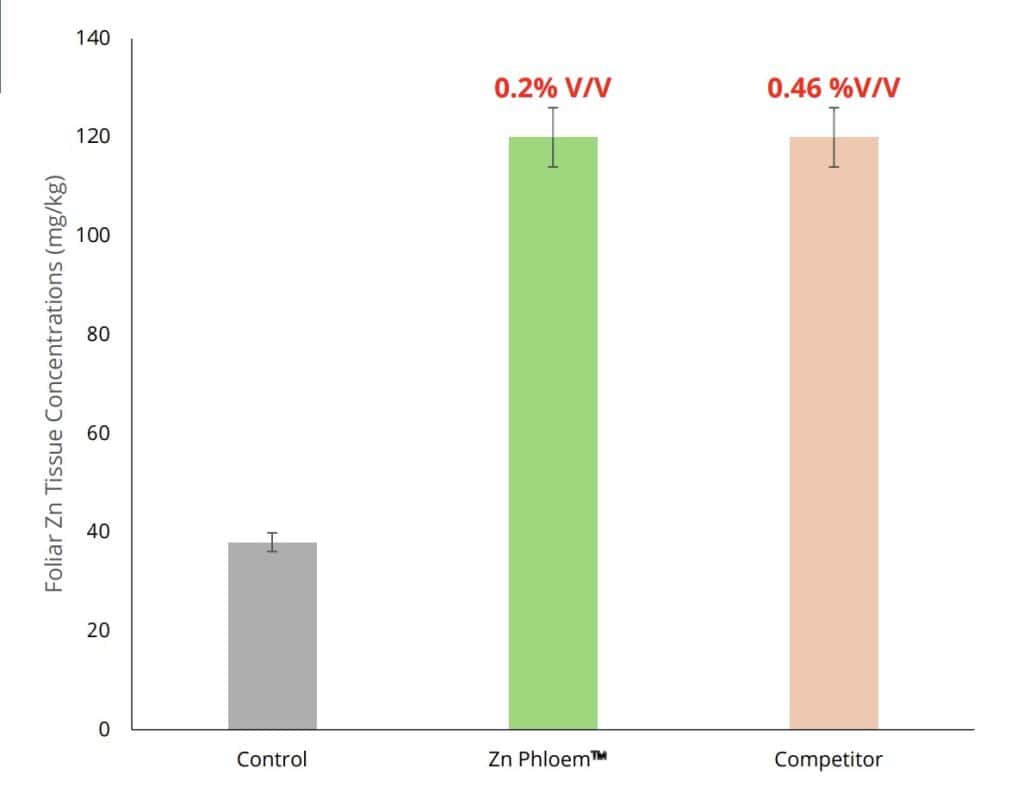
Figure 1: Foliar Zn tissue concentration (mg/kg) of various applied Zn sources, including an untreated control on butterhead lettuce.
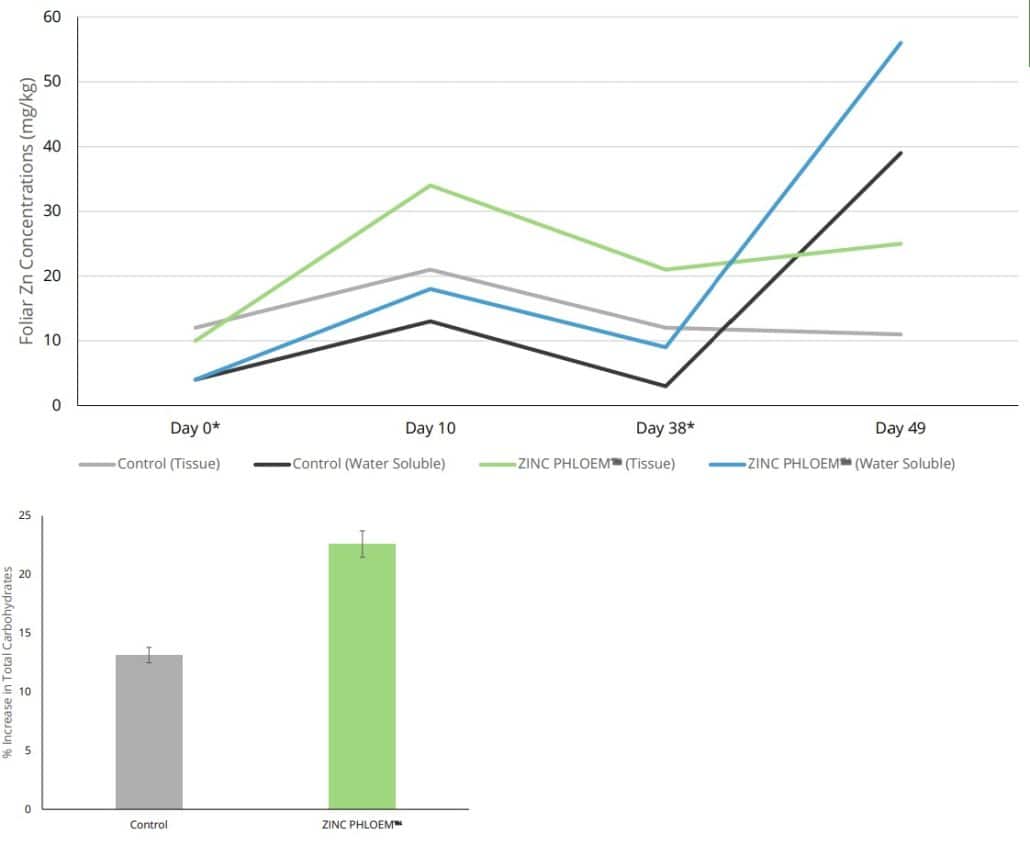
Figures 2A and 2B: Foliar tissue and water-soluble Zn content (mg/kg) of macadamia trees after two applications of ZINC PHLOEM™ on day 0 (*) as well as 38 (*).
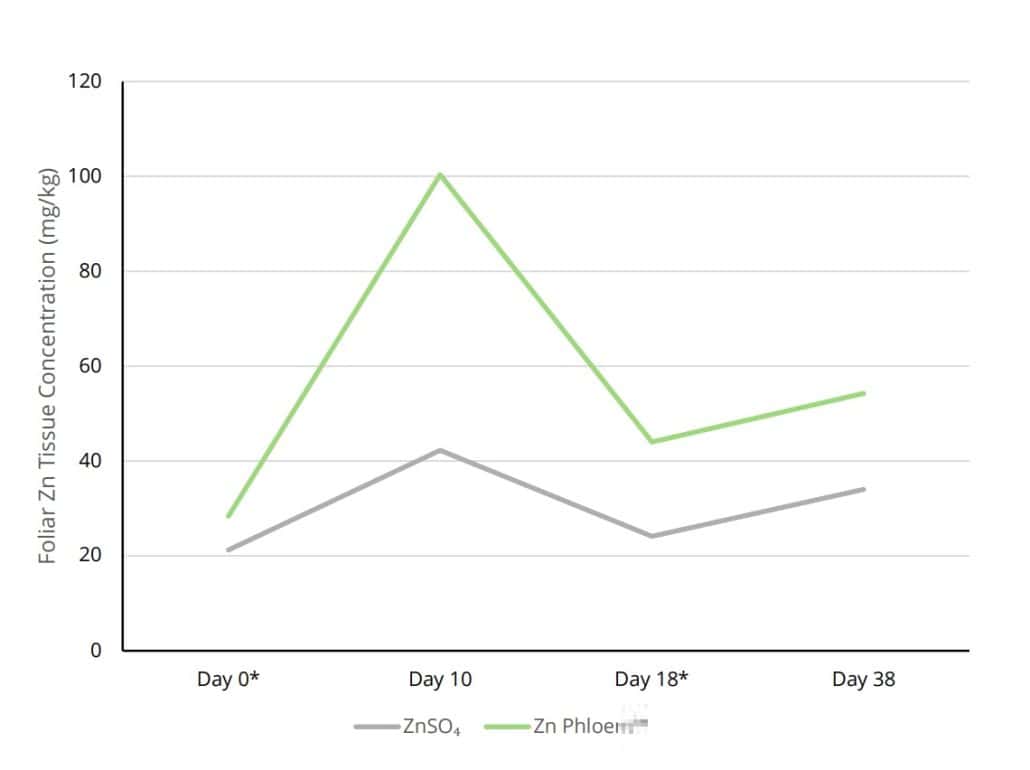
Figure 3: Foliar tissue Zn content (mg/kg) of avocado trees after two applications of ZINC PHLOEM™ on day (*) as well as 18 (*), when compared to ZnSO4 application.
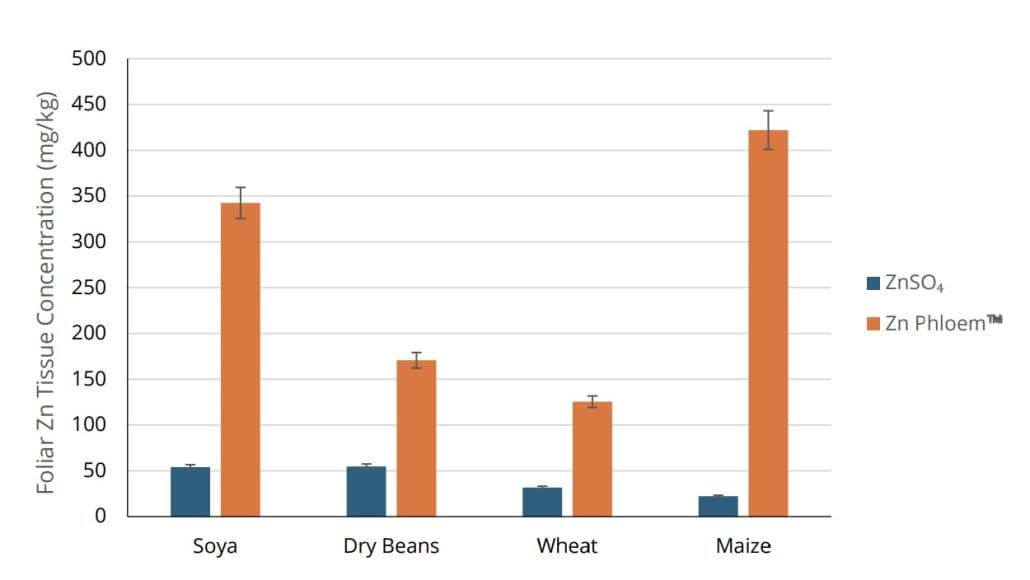
Figure 4: Percentage increase (%) in foliar tissue Zn content of maize, soybean, and wheat plants treated with ZINC PHLOEM™ compared to ZnSO₄.
In addition to leaf samples, carbohydrate samples were collected before and 10 days after application (Figures 2A, 2B).
Results indicate that applying ZINC PHLOEM™ positively influenced the carbohydrate status of macadamia trees (Figures 2A, 2B). Carbohydrate status is a strong indicator of a tree’s ability to set and sustain flowers and fruit. A low carbohydrate status can lead to early flower and fruit/nut abscission due to insufficient reserves to support the high respiratory demand of developing organs. Carbohydrate status also reflects photosynthetic efficiency in producing sugars, which are later used or stored as starch. Low carbohydrate levels have been linked to alternate bearing cycles, resulting in significant yield fluctuations between on- and off-years.
Similarly, avocado trees treated with ZINC PHLOEM™ exhibited increased leaf Zn levels compared to those treated with ZnSO₄ (Figure 3).
ZINC PHLOEM™ is effective across various crop groups and leaf structures (Figure 4). It increased foliar tissue Zn concentrations by 38% in maize, 10% in soybean, and 26% in wheat compared to standard ZnSO₄ applications (Figure 4).
Conclusion
ZINC PHLOEM™ from Agri Technovation ensures efficient zinc foliar uptake, translocation, and assimilation across multiple crop species with diverse leaf morphologies.
Organically complexed and formulated with nutritional synergists, ZINC PHLOEM™ enhances nutrient delivery and absorption, overcoming the limitations typically associated with foliar applications.
Fertilizer Group 2, Reg. No. B5734, Act 36 of 1947. Zinc (Zn): 204,400 mg/L.
References
Xie, R., Zhao, J., Lu, L., et al. (2020). Penetration of foliar-applied Zn and its impact on apple plant nutrition status: In vivo evaluation by synchrotron-based X-ray fluorescence microscopy. Horticulture Research, 7(147).
Yumei, Li, P., Mulligan, D., & Huang, L. (2014). Foliar zinc uptake process and critical factors influencing foliar Zn efficacy. Biointerface Research in Applied Chemistry, 4, 754–766.
Marešová, J., Remenárová, L., Horník, M., et al. (2012). Foliar uptake of zinc by vascular plants: Radiometric study. Journal of Radioanalytical and Nuclear Chemistry, 292, 1329–1337.
Khan, M.R., Akram, M.S., Moonmoon, J.F., Tarafder, M.M.A., Rahman, M.H., Das, S., & Hossain, A. (2023). Soil and foliar zinc application techniques influence productivity, zinc concentration, and protein content in the grains of bread wheat varieties. Acta Agrobotanica, 76, 1–13.
Schreiber, L., & Schönherr, J. (2009). Penetration of ionic solutes. In Water and Solute Permeability of Plant Cuticles (pp. 125–144). Springer.
Schreiber, L. (2005). Polar paths of diffusion across plant cuticles: New evidence for an old hypothesis. Annals of Botany, 95, 1069–1073.
Schönherr, J. (2006). Characterization of aqueous pores in plant cuticles and permeation of ionic solutes. Journal of Experimental Botany, 57, 2471–2491.
Zhou, S., Chen, S., Yuan, Y., et al. (2015). Influence of humic acid complexation with metal ions on extracellular electron transfer activity. Scientific Reports, 5, 17067.
Li, T., Song, F., Zhang, J., Tian, S., Huang, N., & Xing, B. (2020). Experimental and modeling study of proton and copper binding properties onto fulvic acid fractions using spectroscopic techniques combined with two-dimensional correlation analysis. Environmental Pollution, 256, 113465.1–113465.16.
Boguta, P., & Sokołowska, Z. (2020). Zinc binding to fulvic acids: Assessing the impact of pH, metal concentrations, and chemical properties of fulvic acids on the mechanism and stability of formed soluble complexes. Molecules, 25(6), 1297.
Nikoogoftar-Sedghi, M., Rabiei, V., Razavi, F., et al. (2024). Fulvic acid foliar application: A novel approach enhancing antioxidant capacity and nutritional quality of pistachio (Pistacia vera L.). BMC Plant Biology, 24, 241.
Rietra, R.P.J.J., Heinen, M., Dimkpa, C.O., & Bindraban, P.S. (2017). Effects of nutrient antagonism and synergism on yield and fertilizer use efficiency. Communications in Soil Science and Plant Analysis, 48(16), 1895–1920.





